
At Advanced Accelerator Applications, we are reimagining nuclear medicine and cancer care. Our mission is to transform patients’ lives by developing and delivering targeted radioligand therapies and precision diagnostic solutions for oncology.



Expanding our patient reach in Spain with the new Salamanca site
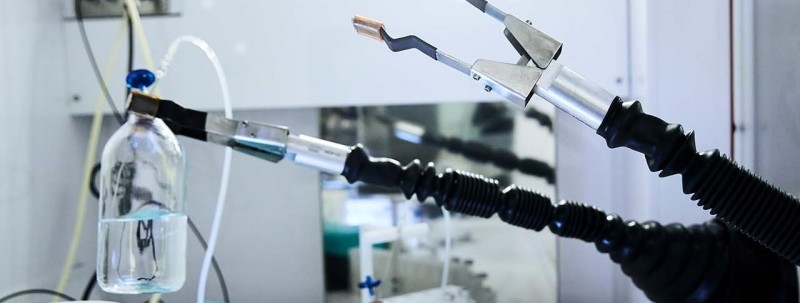
Turning the power of nuclear technology into a medical force

Not your grandparents’ nuclear medicine

Radioligand therapy: delivering now, building for the future
About Advanced Accelerator Applications
+1,000
Associates globally
12
Countries
1 of the 4
platforms of the Novartis Bold4Cure strategy
Founded in 2002, AAA has a strong legacy as a pioneer in nuclear medicine.
We believe targeted radioligand therapy has the potential to be broadly used in many different tumor types.
“This is a very exciting time in AAA’s development. I believe in the potential of radioligand therapy to transform patients’ lives and I am committed to exploring this therapeutic approach in multiple tumor types.”
Sidonie Golombowski-Daffner
President of AAA
